MIMS Editorial Governance & Quality Assurance
1. Overview
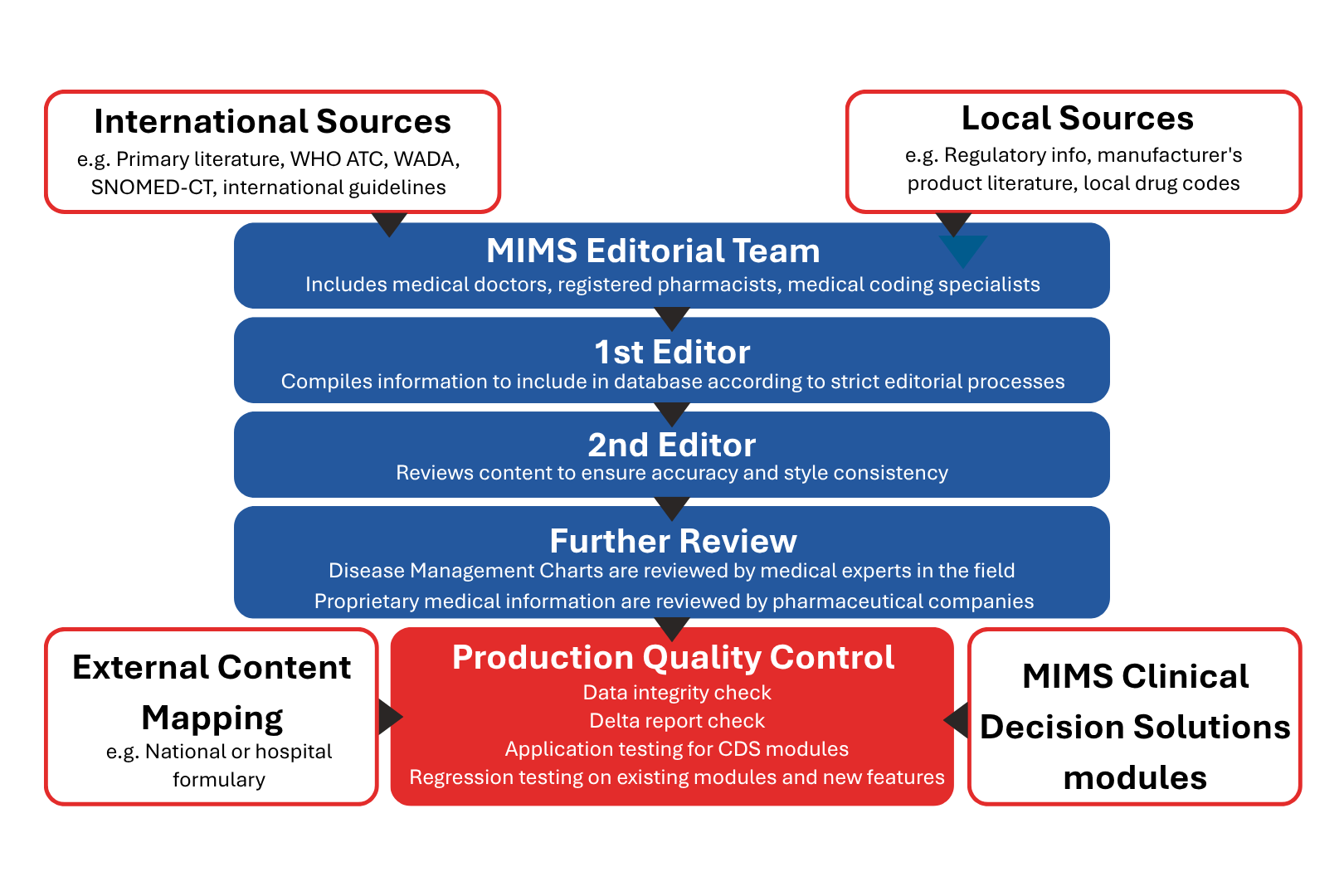
The MIMS Integrated Solution primarily involves editorial work to provide a holistic set of drug information and clinical decision support based on international and local sources. Different processes will be applied to ensure the quality, accuracy, validity, and relevance of the various types of content.
2. Editorial Process for MIMS Drug Product Information
The MIMS Editorial team involved in reviewing and curating the product information or other codified medical information are pharmacists and doctors. We have a rigorous and structured editorial work process in place, dedicated to safeguard quality, accuracy and relevance of medical information. This process has been developed over many years and encompasses a series of checks carried out under strict editorial policy.
The MIMS Editors follow Standard Operating Procedures that ensure that the information is checked by several editors before it is finalised for publishing.
This figure provides a summary of the editorial process for MIMS Drug Product Information.
3. Editorial Process for MIMS Clinical Decision Solutions Modules
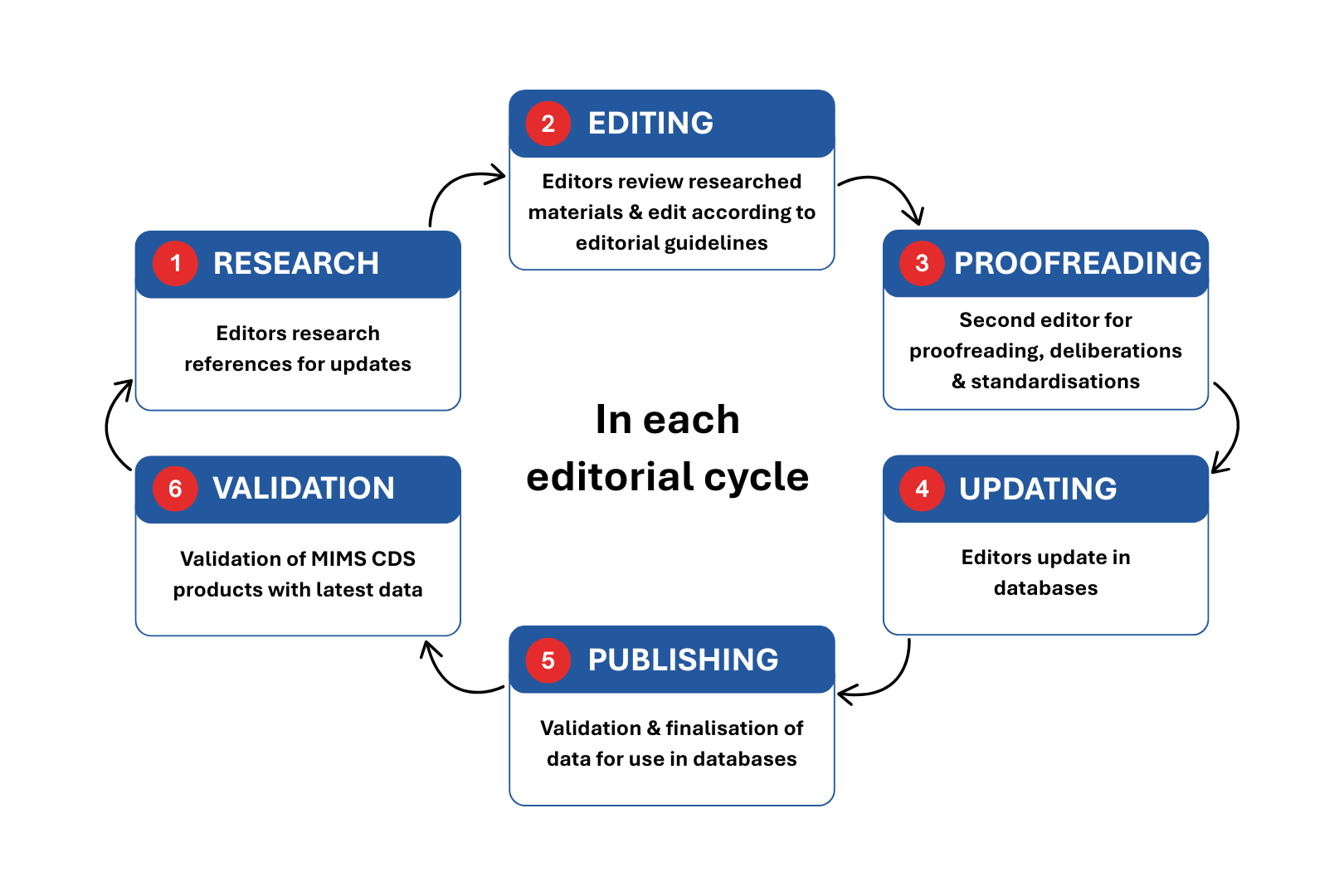
MIMS Clinical Decision Solutions Modules are internationally referenced and clinically reviewed. When integrated into healthcare systems, the clinical decision support modules provide real-time interactivity and intervention checks for doctors and pharmacists, improving medication management at the point of care. The Clinical Decision Solutions Editorial team comprises Clinical Editors and Medical Coding Specialists, who are either Pharmacists or Doctors. The editors research, review and collate information to be included in the database and ensure that the information is accurate and up to date.
To provide healthcare professionals with unbiased evidence-based clinical information, the edits are supported by a thorough review and analysis of international references and important safety alerts from various medication regulatory bodies. All information is subsequently proofed by a second editor before it gets published in the database as per Standard Operating Procedures to ensure consistency and accuracy of the information.
This figure provides a summary of the editorial process for MIMS Clinical Decision Solutions Modules.
4. Production Quality Control
The Editorial team and Quality Assurance team perform regular quality control measures to ensure accuracy of data and functionalities in the respective delivery formats.
Rigorous data checks are conducted within the editorial updating cycle as part of the regular editorial standard operating procedures. The editorial team will deliberate and verify against international references to support clinical practices and improve patient safety. Emphasis is on producing evidence-based drug information for clinicians.
Each delivery file is thoroughly tested by a dedicated team of quality assurance testers on various functionalities before release. The team follows a quality assurance checklist and test the functionalities against the latest test cases as part of the routine testing process.
The quality assurance testing includes the following:
- Data integrity check
- Delta report check
- Application testing for clinical decision support modules
- Regression testing on existing functionalities, as well as new features
New test cases are constantly added, and existing test cases are updated where applicable. The Quality Assurance Manager will review and sign off the quality assurance checklist before approving the release of respective delivery files to the clients.
Try MIMS Clinical Decision Solutions now!
Discover how we empowers you with safer medications and better patient care.
